Genomics and Precision Health Blog – Archive Posts
Population Genomic Screening is Here: We Need Evidence on Health Impact and Optimal Implementation

A recent study identified 12 population-based genomic screening programs in the United States and described their implementation logistics and potential health impact. In the past decade, the promise of genomic screening in the general population has garnered increasing interest due to a combination of factors such as enhanced sequencing capabilities, lowered costs of testing, and Read More >
Posted on by 2 CommentsCan’t Stop, Won’t Stop: The Resiliency of Newborn Screening Programs during the COVID-19 Pandemic
A recent article identified the impact of the COVID-19 pandemic on the newborn screening system and highlighted the importance of collaboration and technical assistance to ensure ongoing operations of this essential public health service. The ongoing COVID-19 pandemic stressed, disrupted, and fundamentally changed the public health system in the United States. While much of the Read More >
Posted on byGenomics and Health Equity: Reaching Asian American, Native Hawaiian, and Pacific Islander Communities

On April 29, 2022, President Biden proclaimed May as Asian American, Native Hawaiian, and Pacific Islander Heritage month to “recognize the innumerable contributions, vibrant cultures, and rich heritage” of Asian Americans, Native Hawaiians, and Pacific Islanders (AA and NHPIs). In addition, the proclamation highlights the Administration’s work reducing poverty among AA and NHPI families and Read More >
Posted on byUsing Implementation Science Frameworks in Genomics and Precision Medicine: We Can Do Better!

A recent scoping review identified many structured approaches to the implementation of genomics and precision medicine and limited use of implementation science frameworks. With continuous advances in genomics and accelerated translation from discovery into clinical practice, in our blog posts, we have repeatedly examined the crucial importance and emerging role of implementation science in the Read More >
Posted on byWhat is the Role of Public Health in Addressing Health Equity in Genomics and Precision Medicine?

The following are excerpts from our recent paper in Genetics in Medicine. Although recent articles have included strong calls for a health equity agenda in genomics and precision medicine, these calls usually focus on underrepresentation of minority and ethnic populations in research. However, to ensure that genomic discoveries can lead to improved population health outcomes, Read More >
Posted on by 1 CommentColliding with Collider Bias: Implications for Precision Public Health
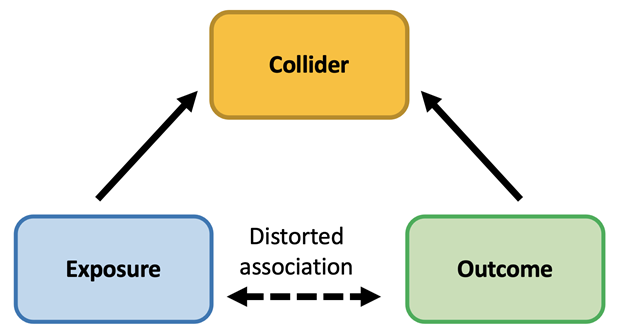
A recent JAMA Guide to Statistics and Methods reviews how collider bias can lead to erroneous inference on causal relationships in clinical and epidemiological studies, potentially leading to incorrect clinical decision making and ineffective public health action. What is Collider Bias? Informed decision making in medicine and public health relies on valid evidence from clinical Read More >
Posted on byHost Genomics and COVID-19: Two Years Later
Early in the COVID-19 pandemic, we explored the rationale for host genomic studies to our understanding of COVID-19 occurrence and outcomes. Two years into the pandemic, we are taking another look. Many academic research groups and consortia—such as the COVID-19 Host Genetics Initiative (COVID-19 HGI) and COVID Human Genetic Effort—have launched worldwide open-science collaborations, featuring Read More >
Posted on byUsing Pharmacogenomics to Better Understand the Role of Selected Medications and Birth Defect Risk

Through a funding opportunity from CDC’s Office of Genomics and Precision Public Health in collaboration with the Office of Advanced Molecular Detection, CDC’s Birth Defects Monitoring and Research Branch in the National Center on Birth Defects and Developmental Disabilities will conduct a 2-year project to gather genome-wide genotyping data to look at relationships between pharmacogenomic Read More >
Posted on byTracking the Scientific Literature on the Impact of Pharmacogenomics on Clinical Practice and Public Health
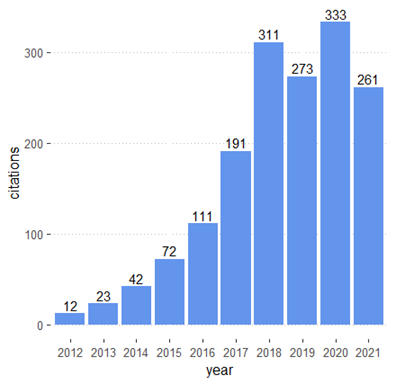
Pharmacogenomics (PGx) is an emerging field that investigates genetic differences in drug effectiveness and safety. PGx is an essential component of precision medicine and seeks to provide the right medication for the right person at the right time. Advances in PGx promise to improve treatment of many diseases such as cancer and cardiovascular disease. In Read More >
Posted on byPrecision Public Health in Action: Enhancing models to predict risk of adverse treatment outcomes in people with hemophilia
In collaboration with the CDC Office of Advanced Molecular Detection, the Office of Genomics and Precision Public Health recently funded the National Center on Birth Defects and Developmental Disabilities (NCBDDD) to strengthen public health capacity by introducing elements of human genomics into applied research on predicting inhibitor risk in people with hemophilia. Hemophilia refers to a group Read More >
Posted on by

