Tracking the Scientific Literature on the Impact of Pharmacogenomics on Clinical Practice and Public Health
Posted on by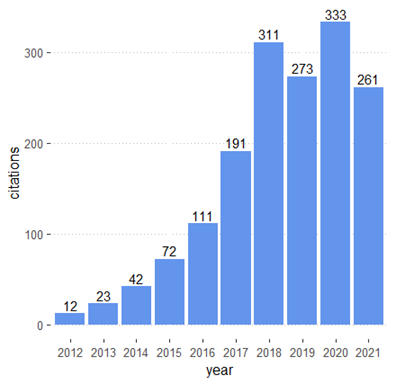
Pharmacogenomics (PGx) is an emerging field that investigates genetic differences in drug effectiveness and safety. PGx is an essential component of precision medicine and seeks to provide the right medication for the right person at the right time. Advances in PGx promise to improve treatment of many diseases such as cancer and cardiovascular disease.
In the last decade, there have been major advances in PGx research. A rapid increase of epidemiologic, translational, and implementation studies have led to the development of guidelines and increased use of PGx in clinical practice. As a result, there has been an increase in guidelines from the Clinical Pharmacogenetics Implementation Consortium (CPIC®). To date, CPIC has published 26 clinical practice guidelines addressing a multitude of gene-drug interactions.
The CDC’s Office of Genomics and Precision Public Health has created a comprehensive resource to help researchers, providers, and policy makers track progress and impact of genomics and precision health on population health. The resource features a continuously updated and curated database of scientific literature and other information. One component of this resource is a specific PGx database that focuses on progress in genomics and precision health technologies in relation to the use of medications in clinical practice and population health.
A quick examination of our PGx database shows significant growth in translation and implementation science in the last decade. The number of published studies in these areas increased from 12 in 2012 to 333 in 2021 (Figure 1). Table 1 provides an overview of some of the most studied drugs and pharmacogenes.
As an example, warfarin is the most cited therapeutic in relation to the genes CYP2C9 and VKORC1. Warfarin is an anticoagulant and blood thinner used to treat thrombotic disorders. The CYP2C9 and VKORC1 genes play a key role in the metabolism of warfarin. Many studies have found evidence that variants in CYP2C9 and VKORC1 can impact the efficacy of warfarin in individual patients. Evidence from numerous large-scale implementation studies has led to the addition of the impact of these genotypes on US Food and Drug Administration) drug labels, as well as clinical guidelines written by CPIC and other groups.
| Gene | ||||
| Therapeutics | CYP2C9 | CYP2C19 | VKORC1 | CYP2D6 |
| Warfarin | 54 | 8 | 54 | 7 |
| Clopidogrel | 9 | 70 | 5 | 9 |
| Tamoxifen | 1 | 3 | – | 30 |
| Citalopram | 2 | 6 | 1 | 6 |
In spite of the growth of PGx studies, the evidence base for clinical utility of PGx tests is still evolving. As such, PGx tests are still not widely used in routine clinical practice despite their potential applications for multiple commonly used drugs. The CDC ACCE framework (analytic validity, clinical validity, clinical utility, and ethical, legal, and social issues) is a useful framework for evaluating the evidence for PGx testing in practice. This framework was proposed as an approach to evaluate the balance of benefits and harms of genetic tests, including PGx, for specific intended uses. It has been used by the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) working group, a CDC-sponsored working group to evaluate the performance of several genetic tests.
Finally, from a health equity perspective, it is important to improve diversity in PGx studies. To date, most genetic research, including PGx studies, has been conducted primarily on individuals of European ancestry. The lack of diversity and racial/ethnic representation in these studies has created a gap in knowledge about the distribution and importance of genetic variants that could be used in practice, potentially leading to inaccurate information on clinical validity and less utility in diverse populations. An ongoing CDC project will measure the prevalence of PGx variation in 150 pharmacogenes in a diverse US population, using 5,000 DNA samples from the population-based NHANES. These data will be used as population reference to evaluate clinical validity and associations of PGx variants with health outcomes in various populations.
Despite ongoing challenges, the increasing number of studies on PGx validity and utility will have a significant impact on clinical practice and population health. We invite our readers to regularly check out our PGx database resources for ongoing advances in the field, including the latest implementation studies, tools and resources, and evidence-based guidelines.
Please submit your comments here.
Posted on by


