When Nanoparticles Blow Up— Explosion Hazards of Nanoparticles
Posted on byThis blog is part of a series to commemorate the 20th anniversary of the Nanotechnology Research Center. Click here for additional blogs in the series and on other nanotechnology topics.
Engineered nanoparticles are synthesized materials with at least one dimension smaller than 100 nanometers. Reactive nanoparticles pose a special hazard. The heat released by the rapid oxidation of aerosolized combustible nanoparticles increases the temperature and pressure of the suspending gas. The resultant destructive pressure forces can damage or destroy structures and endanger nearby personnel. When manufactured on an industrial scale, engineered nanomaterials may thus present an explosion hazard.
Very little is known about the potential explosivity of any material when subdivided down to the nanoscale. This lack of knowledge prompted the NIOSH Nanotechnology Research Center (NTRC) to study the explosivity of several classes of nanoparticles: nanocarbons, nanocellulose, nanometals.
 While only a few nanomaterials are currently produced on an industrial scale, the history of catastrophic consequences from dust explosions involving larger particles (such as coal dust, grain dust or manufactured combustible fine powders) motivates assessing nanomaterials for their dust explosion risk. The photo at left is an aerial view of the remnants of the Imperial Sugar facility following the 2008 sugar dust explosion.
While only a few nanomaterials are currently produced on an industrial scale, the history of catastrophic consequences from dust explosions involving larger particles (such as coal dust, grain dust or manufactured combustible fine powders) motivates assessing nanomaterials for their dust explosion risk. The photo at left is an aerial view of the remnants of the Imperial Sugar facility following the 2008 sugar dust explosion.
 The open Hartmann tube is used to screen dusts for potential explosion hazard and to measure MIE. Shown at right is a dust explosion of single-walled carbon nanotubes (SWCNTs).
The open Hartmann tube is used to screen dusts for potential explosion hazard and to measure MIE. Shown at right is a dust explosion of single-walled carbon nanotubes (SWCNTs).
Quantitative explosion measurements are performed in the Siwek 20-L explosivity chamber (shown below). This apparatus has been extensively used at NIOSH to study the explosion hazards of coal dusts. NIOSH has used the same apparatus for nanoparticle explosion measurements.

The powder is placed at the bottom of the chamber and dispersed by a short blast of dry air. The electrically activated ignition sources, equivalent to the energy in a book of 20 pocket matches, ignite the dust at the center of the chamber; a subsonic (deflagration) flame front propagates outward.
The hazard associated with dust explosions depends on the dispersibility of the powder. NIOSH has studied the dispersibility of a wide variety of nanomaterials [Evans et al., 2013]. The dust, which serves as the fuel for the explosion, is often confined (usually inside equipment, or in air ducts, or within the manufacturing facility), which allows the dust concentration to build up to levels that can support an explosion.
Explosion sensitivity indicates how easy it is to initiate the explosion. The dust can be ignited by a spark, flame, or hot surface. The local temperature of friction, welding, or electrical sparks often exceeds the minimum ignition temperature. The key parameter for spark ignition is the minimum ignition energy (MIE). Below a minimum explosive concentration (MEC) the dust cloud can no longer support an explosion (due to insufficient fuel). Ignition is thus more likely to occur if the dust has a low ignition temperature, a low ignition energy, or a low minimum explosive concentration.
The severity, or violence, of a dust explosion is measured by the maximum pressure developed, and the rate of pressure buildup.
NIOSH Research
Carbonaceous Nanoparticles
NIOSH screened a variety of carbonaceous nanomaterials for potential explosion hazard [Turkevich et al., 2016]:
- single-walled carbon nanotubes
- multi-walled carbon nanotubes
- carbon nanofibers
- graphene
- diamond
- fullerene
These nanomaterials are ‘weakly explosive’ (European Dust Explosion Class St-1), similar to cotton and wood dust. The explosion severity did not depend on primary particle size.
NIOSH also measured the minimum explosive concentration (MEC), minimum ignition energy (MIE), and minimum ignition temperature for these carbon nanopowders [Turkevich et al., 2015]. The nanocarbon MEC is significantly lower than coal dust, carbon black or graphite MEC. Nanocarbons have a higher MIE than coal dust but a lower MIE than fine particle graphites. MIE identification using electrical spark discharge is frustrated by powder conductivity, which may short out the electrical ignition; this raises the additional safety concern of using these materials in the presence of electrical equipment (e.g., high voltage or unshielded circuit boards). The nanocarbons exhibit minimum ignition temperatures, comparable to coal dust and carbon blacks.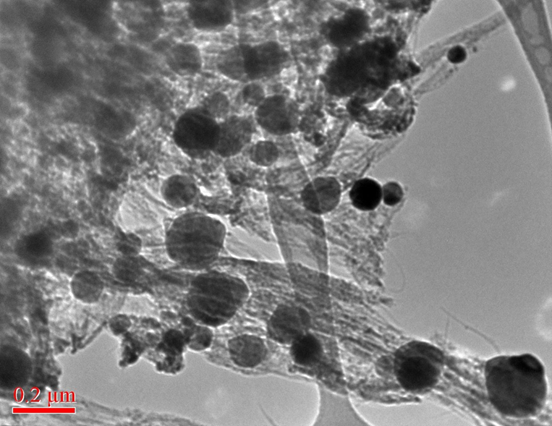
The combustion mechanism appears to be the same for all forms of carbon: carbon atoms are ‘boiled off’ of the solid particles, and high temperature oxidation occurs in the gas phase, yielding carbon monoxide; as the system cools, the carbon monoxide disproportionates, condensing into the soot balls commonly observed in the electron micrographs (shown at right) of the exploded material.
Nanocellulose
Nanocellulose (both nanocrystalline and nanofibrillated) is ‘strongly explosive’ (European Dust Explosion Class St-2), similar to wood flour. The smaller particle size appears not to alter either the combustion mechanism or the explosion parameters.
Nanometals
NIOSH screened [Dastidar et al., 2013] several nanometals (elements colored red and green in the periodic table below). The red elements present an elevated hazard; the green elements are less hazardous.
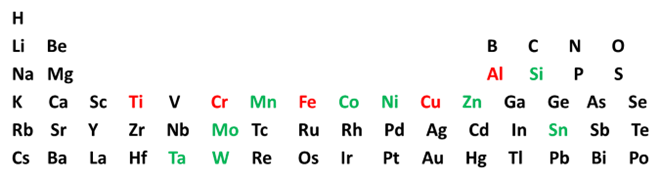
The nanometal oxidation reaction occurs at the particle surface. The higher energy released drives more severe explosions; aluminum and titanium are ‘very strongly explosive’ (European Dust Explosion Class St-3). Because oxidation is so favorable, these materials are intrinsically susceptible to explosion (very low MIE). Pre-existing oxide layers on the particle surfaces inhibit the reaction; thus, these materials may initially not appear to be explosive. However, they can explode violently under transport or processing flow (where frictional heating promotes oxidation). Further study is needed on the explosion characteristics of these industrially important but potentially dangerous nanometals, especially systematically quantifying the passivating role of surface oxides.
Summary
The NIOSH investigation of the explosivity of nanomaterials showed that they behave similarly to the same materials with larger particle sizes. Carbon nanomaterials are weakly explosive (St-1), while nanocellulose is more explosive (St-2). Some nanometals, especially aluminum and titanium, are significantly more explosive (St-3). Pre-existing oxide layers on nanometals may obscure the hazard of explosion, which can nonetheless develop when these materials are transported or processed.
Combustibility should continue to be studied as new and more advanced materials are developed and used in commerce. Manufacturers, suppliers, and end-users should consider these combustibility characteristics when working with nanomaterials to prevent explosions and keep workers safe.
Leonid A. Turkevich, Ph.D., is a Senior Service Fellow in NIOSH’s Division of Field Studies and Engineering/Engineering and Physical Hazards Branch. He is a Fellow of the American Physical Society and of the American Association for the Advancement of Science.
References
D.E. Evans, L.A. Turkevich, C.T. Roettgers, G.J. Deye, P.A. Baron, “Dustiness of Fine and Nanoscale Powders”, Annals of Occupational Hygiene 57, 261-277 (2013).
A.G. Dastidar, S. Boilard, P.R. Amyotte, L.A. Turkevich, “Explosibility of Nano-Sized Metal Powders”. Proceedings of the 9th Global Congress on Process Safety (28 April-1 May 2013, San Antonio, TX)., ed. by V. Edward, L. Turci, J. Chen, J. Shah (American Institute of Chemical Engineers, NY, 2013), paper 47b: 1-17.
L.A. Turkevich, J.E. Fernback, A.G. Dastidar, P. Osterberg, “Potential Explosion Hazard of Carbonaceous Nanoparticles: Screening of Allotropes”, Combustion and Flame 167: 218-227 (2016).
L.A. Turkevich, A.G. Dastidar, Z, Hachmeister, M. Lim, “Potential Explosion Hazard of Carbonaceous Nanoparticles: Explosion Parameters of Selected Materials”, J. Hazardous Materials; 295: 97-103 (2015).
Posted on by

