Respiratory Protection Week 2023: Filling in the Gaps
Posted on by We’re back for another Respiratory Protection Week! This year we’re shining some light on our Respirator Approval Program’s efforts to fill in gaps related to respiratory protection and answering some of your remaining questions about NIOSH Approved® respirators.
We’re back for another Respiratory Protection Week! This year we’re shining some light on our Respirator Approval Program’s efforts to fill in gaps related to respiratory protection and answering some of your remaining questions about NIOSH Approved® respirators.
As you may know, the National Institute for Occupational Safety and Health (NIOSH) is the federal institute responsible for the evaluation and approval of respirators used in U.S. occupational settings. NIOSH’s National Personal Protective Technology Laboratory (NPPTL) mission is to prevent disease, injury, and death for the millions of workers who rely on personal protective technology. One of NPPTL’s main responsibilities is to carry out NIOSH’s respirator evaluation and testing duties for the Respirator Approval Program. The Respirator Approval Program ensures that all NIOSH Approved respirators meet the general construction, strict performance, and quality assurance requirements laid out in the federal regulation 42 CFR Part 84. As you can imagine, these past few years have been very busy.
NIOSH Respirator Approval Program Efforts to Fill Respiratory Protection Gaps
When respirator supply shortages occurred during the beginning of the COVID-19 pandemic, the Respirator Approval Program received an influx of approval requests and questions about the NIOSH approval process. The program worked tirelessly to improve the supply and availability of NIOSH Approved respirators, more than doubling its approval decisions in 2020. Many new manufacturers, designers, and distributors sought NIOSH approval to help combat the shortages. But, as we’ve said before, the approval process isn’t just a one-time test, it’s a detailed process that involves quality control plans, pre-submission test data, respirator samples, and more. There are several steps applicants need to take before they can even submit their product for approval. While reviewing applications from prospective applicants who were new to the approval process, the Respirator Approval Program discovered it needed a way to easily communicate the requirements needed to achieve NIOSH approval to better prepare potential applicants for the process. This need led to the development of our new booklet which outlines everything manufacturers need to know before they apply.
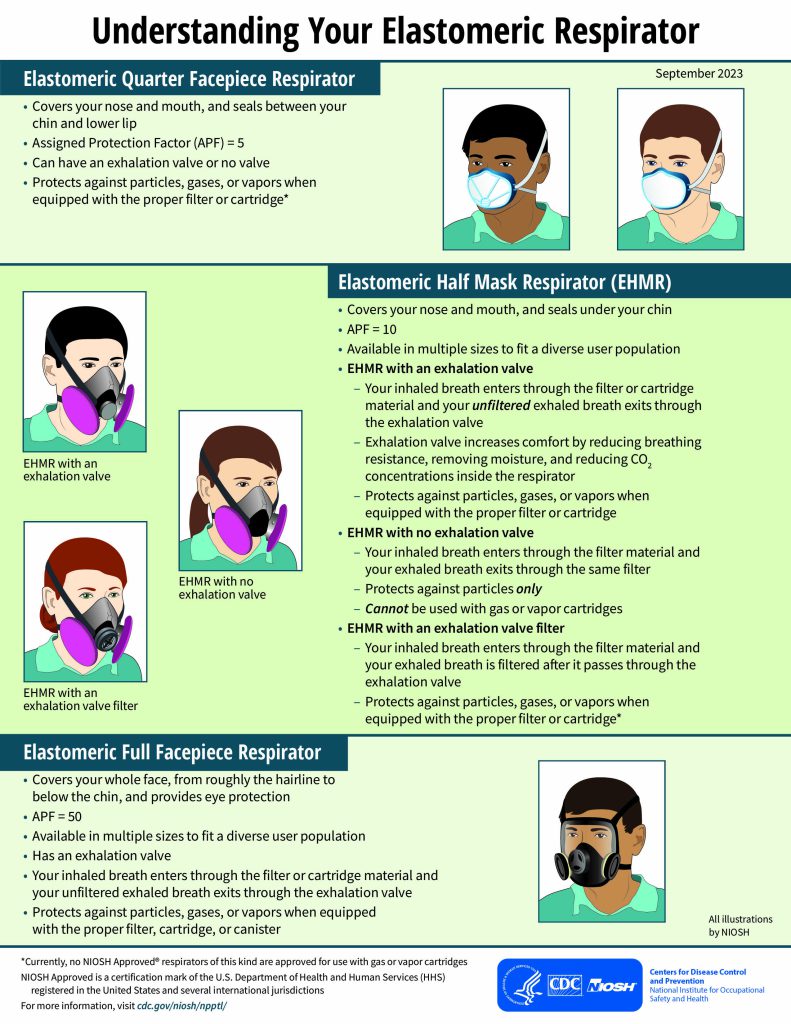 Over the past few years, the Respirator Approval Program also helped address concerns about respirators with exhalation valves by approving innovative elastomeric half mask respirator (EHMR) designs that filtered exhaled breath or had no exhalation valve. During supply shortages, EHMRs are frequently used to supplement the supply of N95® filtering facepiece respirators (FFRs). However, these reusable respirators typically have exhalation valves creating concerns about using them in certain occupational settings. Exhalation valves allow unfiltered exhaled breath to escape through the valve. Therefore, these respirators cannot be used in settings like an operating room which requires a sterile field. Several manufacturers sought approval for EHMR designs with no valve or a valve filter, many were approved and today there are several different options to choose from on our Certified Equipment List (CEL), the official listing of NIOSH Approved products. You can easily find the approved non-valved EHMRs using the CEL’s quick search function! We also created a new infographic (see image at right) to help you learn more about these respirators and the different elastomeric respirator options available to workers.
Over the past few years, the Respirator Approval Program also helped address concerns about respirators with exhalation valves by approving innovative elastomeric half mask respirator (EHMR) designs that filtered exhaled breath or had no exhalation valve. During supply shortages, EHMRs are frequently used to supplement the supply of N95® filtering facepiece respirators (FFRs). However, these reusable respirators typically have exhalation valves creating concerns about using them in certain occupational settings. Exhalation valves allow unfiltered exhaled breath to escape through the valve. Therefore, these respirators cannot be used in settings like an operating room which requires a sterile field. Several manufacturers sought approval for EHMR designs with no valve or a valve filter, many were approved and today there are several different options to choose from on our Certified Equipment List (CEL), the official listing of NIOSH Approved products. You can easily find the approved non-valved EHMRs using the CEL’s quick search function! We also created a new infographic (see image at right) to help you learn more about these respirators and the different elastomeric respirator options available to workers.
Frequently Asked Questions About NIOSH Approved Respirators
While we’re on the topic of respirator approvals and highlighting proper respiratory protection practices for Respiratory Protection Week, we thought this would be the perfect opportunity to answer some frequently asked questions about NIOSH Approved respirators.
Can workers use respirators with revoked or rescinded approval numbers?
When a NIOSH approval is revoked or rescinded, respirators containing the revoked or rescinded approval number can no longer be manufactured, assembled, sold, or distributed as a NIOSH Approved product. NIOSH strives to release a Conformity Assessment Letter to Manufacturers whenever a revocation or rescission occurs to inform users of the affected approval number(s). All revoked or rescinded approvals are removed from our CEL. So, if an approval is not listed in the CEL, it’s no longer approved by NIOSH and shouldn’t be used in workplaces where a NIOSH Approved respirator is required.
What is the shelf life or expiration date of NIOSH Approved respirators?
As you know, there are a lot of requirements that manufacturers must meet to achieve NIOSH approval, but there are some decisions that are left up to them. NIOSH does not require approval holders (manufacturers) to identify a shelf life or expiration date, but some choose to specify one based on their knowledge of the product’s design. So, any questions about shelf life or expiration date are best directed to the approval holder.
Can NIOSH Approved respirators be modified?
When NIOSH approves a respirator, it is approved as a whole system, not as individual components. Therefore, it’s crucial to keep the respirator in an approved configuration so it can provide the expected level of protection. A respirator’s approval label will specify the components that were used to make up the respirator. If a component needs to be replaced, only the ones listed on the label can be used. If components are mixed and matched or parts not on the label are added, the respirator will no longer be in a NIOSH Approved configuration.
Now that your respirator knowledge is warmed up, we hope you’re ready to explore the new resources we have to offer. Head over to our Respiratory Protection Week webpage to see what’s new this year! You can also follow us on social media using #RespiratorWeek. We are so excited to spend another year celebrating this observance with you!
N95 and NIOSH Approved are certification marks of the U.S. Department of Health and Human Services (HHS) registered in the United States and several international jurisdictions.
Meghan Kiederer, BA, is a Health Communication Specialist for the NIOSH National Personal Protective Technology Laboratory.
Jeffrey Peterson is the Branch Chief of the Conformity Verification and Standards Development Branch in the NIOSH National Personal Protective Technology Laboratory.
Posted on by

