Supplementing the Supply of N95s with Reusable Elastomeric Half Mask Respirators
Posted on byAs we celebrate our annual Respiratory Protection Week Observance this year, we at NIOSH want to show our appreciation to all the workers who use respiratory protection and the manufacturers who develop these products to keep our nation’s workers safe. Whether your role is to heal, protect, create, or construct, we rely on your skills and your willingness to do a job that requires systems to be in place to protect the health and safety of America’s workers. We continue striving to support those who support us all. One vital aspect of this is to continue exploring options to make effective respiratory protection available where it is needed most.
The high demand and limited supply of N95 filtering facepiece respirators (FFRs) during the COVID-19 pandemic have led organizations to rely on other types of respirators, such as reusable elastomeric half mask respirators (EHMRs).
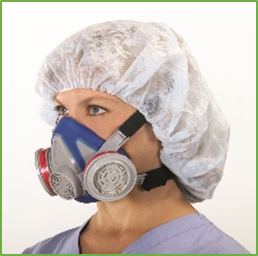
An EHMR is a non-powered NIOSH-approved respirator that has a tight-fitting facepiece that covers the nose and mouth.1 The facepieces are made of synthetic or natural rubber material permitting repeated cleaning, disinfection, storage, and reuse. EHMRs use replaceable filters or cartridges, and they provide the same, or greater, level of protection as single-use N95 FFRs. While EHMRs have been used in many workplace settings for years, their utility in healthcare settings has not been as common.
CDC developed strategies to optimize the supply of EHMRs during conventional and surge demand situations, as experienced during the COVID-19 pandemic.1 NIOSH-approved EHMRs provide an alternative respiratory protection option capable of reducing the total number of respirators required because EHMRs may be cleaned, disinfected, and reused numerous times.1 Unless the EHMR filter cartridges become visibly soiled or wet, visibly damaged, or if the respirator becomes notably harder to breathe through, current practice shows that conservatively, the filters could be used for at least one year.1 Although more popular in industry settings, EHMRs have been leveraged both before and during the COVID-19 public health emergency and have been highlighted in several recent media reports.2-5
Although EHMRs require a higher up-front cost than N95 FFRs, the EHMR facepiece and cartridge reusability may provide cost-savings advantages and may create less hospital waste compared to the disposable N95 FFR.6 For example, due to COVID-19 N95 FFR shortages, one large academic medical center—comprising 12 hospitals—purchased and deployed 10,000 EHMRs that reduced N95 FFR usage to zero.7,8 The center reported a significant cost benefit. The one-time cost and storage of EHMRs was 10 times less expensive after one month of use when compared to disposable N95 FFRs.7,8
Research has shown that user acceptance, fit testing, and disinfection are not barriers to implementing EHMRs.9-12 With proper use, fit, and maintenance training, EHMRs provide an effective solution to supplementing the supply of N95 FFRs.
Generally, EHMRs have exhalation valves, which should be taken into consideration before use in a sterile setting or for use as source control. Until more research is available, masks with exhalation valves or vents should NOT be worn to help prevent the person wearing the mask from spreading COVID-19 to others (source control). Here are some tips when it comes to exhalation valves:
- Wear a respirator without an exhalation valve when both source control and respiratory protection are required.
- If only a respirator with an exhalation valve is available and source control is needed, cover the exhalation valve with a surgical mask, procedure mask, or a cloth mask that does not interfere with the respirator fit.
We are continuing to explore strategies to augment the supply of disposable N95 FFRs by leveraging reusable EHMRs and appropriate filter cartridges. The Strategic National Stockpile (SNS) plans to purchase EHMRs and filter cartridges for storage and distribution. NIOSH is assisting the SNS to develop this nation-wide distribution strategy. In collaboration with the SNS, NIOSH has prepared a Federal Register Notice to request information from the public regarding the deployment and use of EHMRs in healthcare settings and emergency medical services organizations during the COVID-19 crisis.
Additionally, NIOSH, in collaboration with our partners, is developing an EHMR Hospital Implementation Guide and Best Practice Guidelines. This Guide will incorporate research findings and input from experienced EHMR hospital users, so that healthcare organizations who may want to implement EHMRS, but are not experienced with their use, will have information related to EHMR use, training, storage, and cleaning.
For more NIOSH respiratory protection information, see the Respiratory Protection Week webpage.
Update, 14 September 20
We are continuing to explore strategies to augment the supply of disposable N95 FFRs by leveraging reusable EHMRs and appropriate filter cartridges. The Strategic National Stockpile (SNS) plans to purchase EHMRs and filter cartridges for storage and distribution. NIOSH is assisting the SNS to develop this nation-wide distribution strategy. In collaboration with the SNS, NIOSH has prepared a Federal Register Notice to request information from the public regarding the deployment and use of EHMRs in healthcare settings and emergency medical services organizations during the COVID-19 crisis. This Federal Register Notice was posted on 09/14/2020. As such, this blog has been closed to comments. Please consider providing input to the Federal Register Notice (deadline extended to December 14, 2020) by submitting responses to Dr. Lee Greenawald, NIOSH, 626 Cochrans Mill Road, Building 141, Pittsburgh, PA 15236, or ppeconcerns@cdc.gov.
Lee Greenawald, PhD, is a Physical Scientist in the NIOSH National Personal Protective Technology Laboratory.
Jaclyn Krah Cichowicz, MA, is a Health Communications Specialist in the in the NIOSH National Personal Protective Technology Laboratory.
Maryann M. D’Alessandro, PhD, is the Director of the NIOSH National Personal Protective Technology Laboratory.
References
- CDC [2020]. Elastomeric Respirators: Strategies During Conventional and Surge Demand Situations. https://www.cdc.gov/coronavirus/2019-ncov/hcp/elastomeric-respirators-strategy/index.html. April 20, 2020. Accessed 08/23/2020.
- University of Minnesota, Center for Infectious Disease Research and Policy. Exploring Respirator Options for Protecting Health Workers from COVID-19. https://www.cidrap.umn.edu/news-perspective/2020/05/exploring-respirator-options-protecting-health-workers-covid-19. May 15, 2020. Accessed 08/23/2020.
- New York Times. They Evoke Darth Vader, but These Masks May Save Your Doctor’s Life. https://www.nytimes.com/2020/05/27/us/coronavirus-masks-elastomeric-respirators.html. May 27, 2020. Accessed 08/23/2020.
- KENS5. https://www.kens5.com/article/news/health/san-antonio-hospital-could-have-an-answer-to-the-ppe-crisis-elastomeric-masks/273-882e7ea3-e377-4776-906c-33ce89e193cc. May 1, 2020. Accessed 08/23/2020.
- The Times. AHN Receives Respirators that Filter “Nearly All Airborne Particles”. https://www.timesonline.com/news/20200416/ahn-receives-respirators-that-filter-nearly-all-airborne-particulates. April 16, 2020. Accessed 08/23/2020.
- https://blogs.cdc.gov/niosh-science-blog/2017/07/06/elastomerics/
- Sricharan Chalikonda, Hope Waltenbaugh, Sara Angelilli, Tiffany Dumont, Curt Kvasager, Timothy Sauber, Nino Servello, Anil Singh, Rafael Diaz-Garcia. Implementation of an Elastomeric Mask Program as a Strategy to Eliminate Disposable N95 Mask Use and Resterilization: Results from a Large Academic Medical Center. Journal of the American College of Surgeons. Volume 231, Issue 3, 2020,Pages 333-338, ISSN 1072-7515, https://doi.org/10.1016/j.jamcollsurg.2020.05.022.
- American College of Medical Toxicology. An Alternative to Disposable N95s: The Reusable Elastomeric Half-Mask Respirator Experience. https://www.acmt.net/_Elastomeric_Half-Mask_Respirators.html. Accessed 08/23/2020
- Pompei LA, Kraft CS, Brownsword EA [2020]. Training and Fit Testing of Health Care Personnel for Reusable Elastomeric Half-Mask Respirators Compared with Disposable N95 Respirators. JAMA. doi:10.1001/jama.2020.4806
- Bessesen M, Adams JC, Radonovich L, Anderson J [2015]. Disinfection of Reusable Elastomeric Respirators by Health Care Workers: A Feasibility Study and Development of Standard Operating Procedures. American Journal of Infection Control 43(6):629-634.
- CDC [2020]. Coronoavirus Disease 2019 (COVID-19): Decontamination and Reuse of Filtering Facepiece Respirators. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
- Hines, SE, Brown, C, Oliver, M, Gucer, P, Frisch, M, Hogan, R, Roth, T, Chang, J, McDiarmid, M. User acceptance of reusable respirators in healthcare. American Journal of Infection Control. 2019:47:648-655.
Posted on by

