Understanding the Use of Imported Non-NIOSH-Approved Respirators
Posted on byWhen a respirator has been approved by NIOSH, the user can be confident that the device will provide the expected level of protection, as long as it fits properly and is worn correctly. But when serious outbreak conditions cause a shortage of the NIOSH-approved filtering facepiece respirators (FFRs), other reliable options must be found.
When possible, NIOSH recommends the use of reusable elastomeric respirators and powered air-purifying respirators (PAPRs) as alternatives to FFRs. However, when a healthcare facility still needs additional FFRs, employers should understand the additional options that the CDC and FDA have determined are appropriate to protect healthcare workers during the pandemic when respirator shortages are reported across the nation.
On March 24, 2020, in response to the evolving COVID-19 public health emergency and continued shortage of FFRs, FDA issued an Emergency Use Authorization (EUA) to allow the use of filtering facepiece respirators from specific countries where the devices are evaluated using methods similar to those used by NIOSH and are expected to provide adequate protection for healthcare personnel. These countries and regions include Australia, Brazil, Europe, Japan, Korea, and Mexico. FDA issued an update to the EUA on April 3, 2020 to include select respirators manufactured in China.
As part of their Strategies for Optimizing the Supply of N95 Respirators, CDC has provided a list of standards used in the countries listed in the original EUA as well as in China. Devices manufactured by current NIOSH-approval holders, who also produce respirators under standards authorized in other countries, are expected to provide the protection indicated.
More information about this authorization, including international importation questions, can be found in the FDA’s Non-NIOSH Approved Respirator EUA FAQ.
A note for respirator manufacturers from countries not included in the March 24, 2020 FDA EUA or the April 3, 2020 update
Manufacturers from countries whose standards or approval mechanisms are not included under the current FDA EUAs can submit a separate EUA request. For information about what to include and where to submit this request, see the FDA Non-NIOSH Approved Respirator EUA FAQ.
Additional important information about importing respirators can be found on the Customs and Border Protection website.
Determining the Reliability of Imported Respirators
- Devices supplied by current NIOSH approval holders who also produce respirators under the various standards authorized in other countries will provide the advertised level of protection, if a proper fit is achieved.
- Devices developed by manufacturers who are not NIOSH approval holders but who conform to the standards of one of the countries or regions listed in the FDA EUA, should have a certificate of approval from an authorized test laboratory confirming that they meet the standards identified in the CDC Guidance described in the Crisis Contingency Strategy.
- Non-NIOSH-approved products developed by manufacturers who are not NIOSH approval holders (and do not have a certificate of approval from an authorized test laboratory from one of the countries identified within the FDA EUA) should only be used in crisis situations when no NIOSH-approved N95 respirator (or a listed device within the FDA EUA) is available. These devices should not be used during aerosol-generating medical procedures unless the alternative is a loose-fitting surgical mask or improvised device.
Table 1. Other countries’ approved respirators product classifications that are acceptable for use in healthcare settings
|
Country
|
Acceptable Product Classifications |
| Australia | P3
P2 |
| Brazil | PFF3
PFF2 |
| China | KN/KP95 KN/KP100 |
| Europe | FFP3
FFP2 |
| Japan | DS/DL3 DS/DL2 |
| Korea | Special
1st |
| Mexico | N100, P100, R100 N99, P99, R99 N95, P95, R95 |
NIOSH is working to confirm that imported respirators provide the expected level of protection
NIOSH is offering to assess the filtration efficiency of respirators received from non-U.S. countries. Interested parties can send a small sample size of respirators received from other countries to be tested at the NIOSH National Personal Protective Technology Laboratory.
Results from these assessments are showing that some products claiming to conform to the European and Chinese standards do not provide the level of protection expected. While some have filtering efficiencies close to 95%, most are measuring well below 95%. Assessment results can be viewed on the NIOSH website.
NIOSH is also testing respirators from stockpiles and unused (without the respirator being contaminated) respirators that have gone through decontamination cycles in order to provide users with a preliminary filtration assessment.
The purpose of offering this testing is to provide an assessment of the filtration performance of the sample received in an effort to support the health, safety, and psychological well-being of frontline workers during the COVID-19 response. However, this testing will not result in, nor contribute to, the process of obtaining a NIOSH approval for the models assessed.
For more information or to submit a request, see the NIOSH National Personal Protective Technology Laboratory (NPPTL) website.
How to tell if a respirator is NIOSH-approved
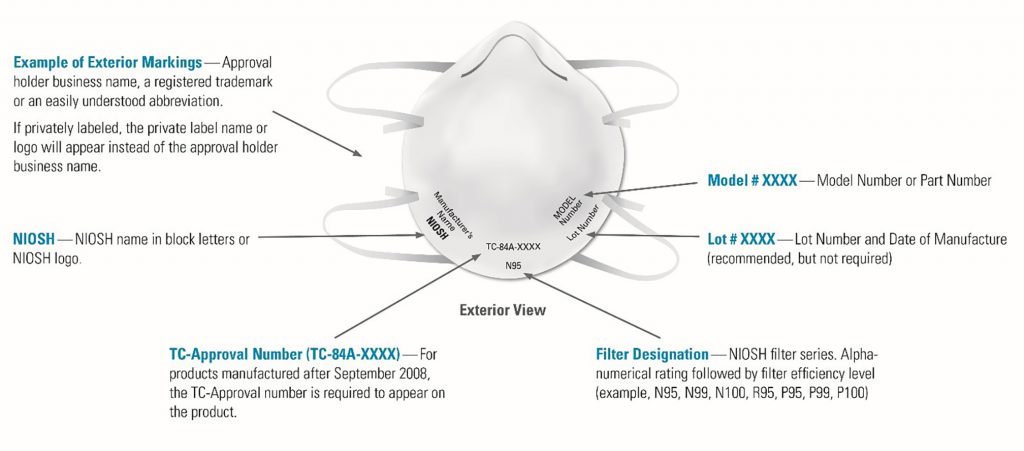
NIOSH-approved respirator models, regardless of their country of origin, are still encouraged for healthcare workers. However, buyers should beware that there are an unprecedented number of falsified claims of NIOSH approval of products on the market due to the current demand, many of which originate from China.
Counterfeit respirators are products that are falsely marketed and sold as being NIOSH-approved and may not be capable of providing appropriate respiratory protection to workers. Inspect the respirator and/or its packaging for the required labeling, as seen in the image above.
The most important marking to verify NIOSH approval is the respirator’s approval number, called its “TC Number.” This number can be verified on the NIOSH List of Approved Filtering Facepiece Respirators or on the NIOSH Certified Equipment List.
Markings on a NIOSH-approved FFR may appear on the facepiece itself or on the straps. If an FFR has approval markings but is not on the NIOSH table of approved filtering facepiece respirators, it is likely to be either a counterfeit product or a respirator that has had its certification revoked or rescinded by NIOSH. If there is no TC Number on the respirator’s packaging, the user instructions, or the product itself, it is not NIOSH-approved. Other clues that a respirator is not NIOSH-approved include the misspelling of “NIOSH,” the presence of decorative fabric or add-ons like sequins, or claims of approval for children. For more information, see the Misrepresentation of NIOSH-Approval webpage.
Maryann M. D’Alessandro, PhD, is the Director of the NIOSH National Personal Protective Technology Laboratory.
John Powers, BS, is the Evaluation and Testing Branch Chief in the NIOSH National Personal Protective Technology Laboratory.
Jaclyn Krah Cichowicz, MA, is a Health Communications Specialist in the in the NIOSH National Personal Protective Technology Laboratory.
The Centers for Disease Control and Prevention is addressing questions related to the Coronavirus Disease 2019 through CDC-INFO and on their webpage. As such, this blog has been closed to comments. Please visit https://www.cdc.gov/coronavirus/2019-ncov/index.html. You can find the most up-to-date information on the outbreak and get the latest answers to frequently asked questions. If you have specific inquiries, please contact CDC-INFO at https://wwwn.cdc.gov/dcs/contactus/form or by calling 800-232-4636. If you have questions about PPE that are not related to Coronavirus Disease 2019, please contact us at PPEConcerns@cdc.gov.
Posted on by

