Category: evidence-based medicine
An Expanding List of Tier 1 Genomic Applications: Evidence-based Guidelines for Hypertrophic Cardiomyopathy and Public Health
The CDC Tier-Classified Guideline Database includes three Tier 1 guidelines on hypertrophic cardiomyopathy (HCM). A 2014 guideline from the European Society of Cardiology, a 2017 guideline from the American Heart Association, American College of Cardiology, and Heart Rhythm Society, and a 2020 guideline from the American Heart Association and American College of Cardiology all recommend Read More >
Posted on by 2 CommentsWork in Progress: Classifying Evidence-based Genomic Applications for Practice and Prevention
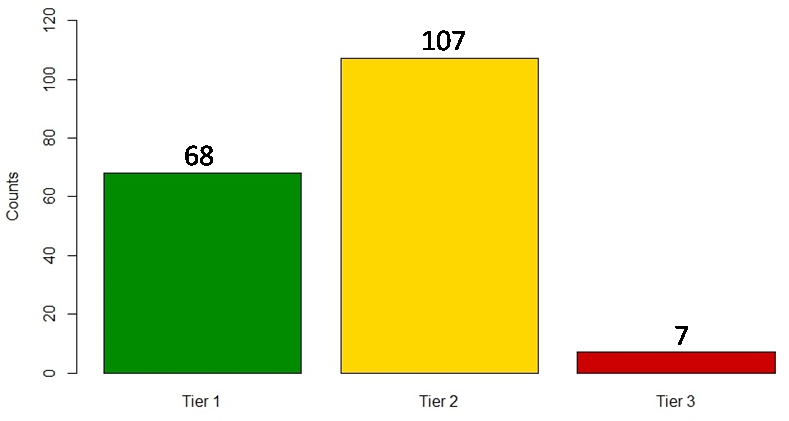
In our 2015 paper,“Prioritizing genomic applications for action by level of evidence: A horizon-scanning method,” we proposed a systematic scanning method that assigns genomic applications to “tiers” defined by availability of synthesized evidence. Because of the amassed evidence on the validity and utility of genomic tests and related technologies, we suggested that researchers, policy makers, Read More >
Posted on byCelebrating a Decade of Evidence-Based Evaluation of Genomic Tests

CDC’s Office of Public Health Genomics (OPHG) launched the Evaluation of Genomic Applications in Practice and Prevention Initiative (EGAPP) in 2004. The independent EGAPP Working Group (EWG) celebrated a decade of achievements and accomplishments at their meeting in Atlanta on October 27-28, 2014. The EWG is comprised entirely of volunteers, encompassing multiples areas of expertise Read More >
Posted on byPublic Health Approach to Big Data in the Age of Genomics: How Can we Separate Signal from Noise?

The term Big Data is used to describe massive volumes of both structured and unstructured data that is so large and complex it is difficult to process and analyze. Examples of big data include the following: diagnostic medical imaging, DNA sequencing and other molecular technologies, environmental exposures, behavioral factors, financial transactions, geographic information & social Read More >
Posted on by 1 CommentIs Evidence-based Medicine the Enemy of Genomic Medicine?

A general practitioner recently writing in the BMJ, said that evidence-based medicine is polluted with “fraud, sham diagnosis, short term data, poor regulation, surrogate ends, questionnaires that can’t be validated, and statistically significant but clinically irrelevant outcomes”, all leading to “overdiagnosis and misery”. In more temperate tones, Goldberger and Buxton recently suggested in a JAMA Read More >
Posted on byNew Products from the EGAPP Working Group: Further Development of Evidence Review Methodology and More Recommendations in Genomic Medicine

The independent EGAPP working group (EWG) held its 27th meeting on May 13-14, 2013 via a virtual online venue. The EWG has been very active since the last meeting. Highlights included: The EWG has four new publications since the last meeting: Recommendations from the EGAPP Working Group: does genomic profiling to assess type 2 diabetes Read More >
Posted on byImplementing Evidence-based Genomics Recommendations at the Intersection of Public Health and Healthcare

We take the opportunity of March 22, 2013, designated as Lynch Syndrome Awareness Day by 13 U.S. state governors and counting, to highlight state public health genomics programs that are taking innovative approaches to implement evidence-based genomic testing recommendations for hereditary cancer syndromes, including Lynch syndrome. Read More >
Posted on byAccelerating the Development of Evidence Reviews and Recommendations in Genomic Medicine

The independent EGAPP working group (EWG) held its 26th meeting on February 11-12, 2013 at the CDC campus in Atlanta. Highlights included: Three EWG recommendation statements on the validity and utility of genetic tests are pending publication on: KRAS, BRAF and other markers involved in EGFR signaling, which are used to inform choice of therapies for Read More >
Posted on byNew Strategies For Public Health Genomics Beyond Newborn Screening

A Working Meeting and an Action Plan to Save Lives Now Nearly 2 million Americans are affected by one of three genetic conditions with a strong risk of early morbidity and mortality: BRCA 1/2 and hereditary breast and ovarian cancer; Lynch syndrome and colorectal , endometrial and ovarian cancer; and familial hypercholesterolemia and early cardiovascular events. At Read More >
Posted on by 2 CommentsEvidence Matters in Genomic Medicine—Round 3: Integrating Family Health History into Clinical Preventive Services
A new podcast from the CDC Expert Commentary Series on Medscape—Family Health History: Use It to Inform Preventive Services for Your Patients— describes how family health history can inform the delivery of preventive health services. The podcast presents three case studies based on recommendations of the US Preventive Services Task Force (USPSTF): screening for lipid Read More >
Posted on by