Category:
Using Pharmacogenomics to Better Understand the Role of Selected Medications and Birth Defect Risk

Through a funding opportunity from CDC’s Office of Genomics and Precision Public Health in collaboration with the Office of Advanced Molecular Detection, CDC’s Birth Defects Monitoring and Research Branch in the National Center on Birth Defects and Developmental Disabilities will conduct a 2-year project to gather genome-wide genotyping data to look at relationships between pharmacogenomic Read More >
Posted on byTracking the Scientific Literature on the Impact of Pharmacogenomics on Clinical Practice and Public Health
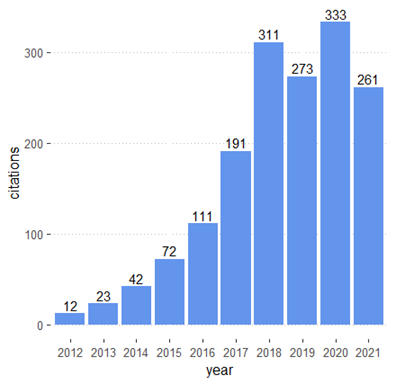
Pharmacogenomics (PGx) is an emerging field that investigates genetic differences in drug effectiveness and safety. PGx is an essential component of precision medicine and seeks to provide the right medication for the right person at the right time. Advances in PGx promise to improve treatment of many diseases such as cancer and cardiovascular disease. In Read More >
Posted on by

