Trends in Tier 1 Genomic Applications 2013-2022
Posted on by The CDC Tier 1 genomic applications database can help consumers, providers, health care organizations and public health programs accelerate the translation of genomic discoveries into improved population health.
The CDC Tier 1 genomic applications database can help consumers, providers, health care organizations and public health programs accelerate the translation of genomic discoveries into improved population health.
Background
Advances in genomics and precision medicine are proceeding at a rapid pace. Many genomic tests have reached clinical practice without clear indication as to whether their use will actually improve health. In order to provide general information to healthcare providers and the general public, in 2014 our office introduced a simple approach for classifying genomic applications based upon 1) type of evidence and 2) recommendation for use of genomic applications. This systematic classification approach assigns genomic applications into one of three tiers.
In a 2019 blog post we introduced a revised CDC Tier-Classified Guidelines Database within the Public Health Genomics and Precision Health Knowledge Base (PHGKB) that reports tier classification levels for guideline documents, rather than of the specific genomic applications or guidance statements included in the guidelines. Guideline documents in the CDC Tier-Classified Guidelines Database include 1) guidelines based upon systematic reviews (SR), 2) U.S. Food & Drug Administration (FDA) cleared or approved companion diagnostic devices or FDA drug labels requiring use of test to inform choice or dose of drug (FDA) and 3) Centers for Medicare & Medicaid Services (CMS) national and local coverage determinations for genetic testing applications that are part of services deemed reasonable and necessary for the diagnosis or treatment of an illness or injury, and within the scope of a Medicare benefit category (CMS).
In this post, we provide a closer look at trends and contents of the publications designated as Tier 1 and briefly summarize their content. We reviewed each guideline document to identify the condition(s)/disease(s) for which the guideline applied. Curation also was performed to identify the focus of the application(s) referenced within the guideline document. These foci included the following:
- Genetic counseling (i.e., pre- and post-genetic test counseling),
- Predictive testing (e.g., testing for at-risk germline mutations),
- Risk management (e.g., surveillance and early detection for disease of at-risk individuals based on mutation status),
- Diagnostic strategies (e.g., somatic alteration testing and differential diagnostic methods, disease screening methods),
- Medical management (i.e., medical treatment for disease/condition),
- Surgical management (i.e., surgical means for disease/condition),
- Pharmaceutical management (i.e., pharmaceutical treatment for disease/condition, testing for resistance or responsiveness to drug treatment),
- Pharmaceutical toxicity (i.e., germline testing for contraindication of drug),
- Prognosis (i.e., testing for disease progression/stage),
- Familial testing (i.e., cascade screening and parent-child trio testing),
- Newborn screening (i.e., germline or biomarker testing in the neonatal period),
- Prenatal testing (i.e., prenatal germline testing).
In addition to coding for disease/condition and application focus, we reviewed each guideline document to identify whether guideline related to germline or somatic genetic testing, or involved another testing method (e.g., family history, biomarkers, cytogenetics). Germline testing is performed to identify an inherited genetic variant that is passed down from one or both parents, or in some cases germline testing will identify a mutation which occurs for the first time in the egg or sperm cell (de novo mutation). Somatic testing is performed to identify random mutations that occur post-conception in individual cells and to date, is most frequently used for cancer genetic testing. Some guideline documents addressed both germline and somatic testing. In other circumstances, the testing method was neither germline or somatic, but used other means for identifying genetic risk (e.g., family history), or diagnosis, including biomarkers (objective measures of a biological state or condition within cells or organisms) and cytogenetics (the study of chromosomes and their inheritance).
What did we find?
Overview
To date, we have identified a total of 132 Tier 1 classified guideline documents published between 2013 and July 2022. An overview of the Tier 1 guideline documents for condition/disease (Table 1), genomic testing type (Table 2) and testing application focus (Table 3), by type of guidance (systematic review guideline, FDA guidance or CMS guidance) are presented below.
Table 1. Breakdown of Tier 1 guideline documents by disease/condition and type of guidance
| Type of guidance | ||||
|---|---|---|---|---|
| Condition/disease area | Systematic Review | FDA | CMS | Total |
| Cancer | 40 | 36 | 31 | 107 |
| Heart, lung, blood | 9 | 2 | 2 | 13 |
| Other | 7 | 1 | 4 | 12 |
| Total | 56 | 39 | 37 | 132 |
Table 2. Breakdown of Tier 1 guideline documents by type of genetic testing and type of guidance (systematic review guideline, FDA guidance or CMS guidance)
| Type of guidance | ||||
|---|---|---|---|---|
| Systematic Review | FDA | CMS | Total | |
| Germline | 20 | 2 | 9 | 31 |
| Somatic | 24 | 24 | 25 | 73 |
| Both | 5 | 9 | 2 | 16 |
| Other | 7 | 4 | 1 | 12 |
| Total | 56 | 39 | 37 | 132 |
Table 3. Breakdown of Tier 1 guideline documents by genetic application focus and type of guidance (systematic review guideline, FDA guidance or CMS guidance).
| Type of guidance | ||||
|---|---|---|---|---|
| Focus Area* | Systematic Review Guidelines | FDA Guidance | CMS Guidance | Total |
| Genetic counseling | 12 | 0 | 0 | 12 |
| Predictive testing | 14 | 0 | 3 | 17 |
| Risk management | 10 | 0 | 4 | 14 |
| Diagnostic strategies | 12 | 0 | 19 | 31 |
| Medical management | 11 | 0 | 3 | 14 |
| Surgical management | 7 | 0 | 5 | 12 |
| Pharmaceutical management | 29 | 39 | 17 | 85 |
| Pharmaceutical toxicity | 1 | 0 | 0 | 1 |
| Prognosis | 3 | 0 | 15 | 18 |
| Familial testing | 8 | 0 | 0 | 8 |
| Newborn screening | 1 | 0 | 0 | 1 |
| Prenatal testing | 2 | 0 | 0 | 2 |
| Total | 110 | 39 | 66 | 215 |
| *Counts are not mutually exclusive for individual guideline documents. | ||||
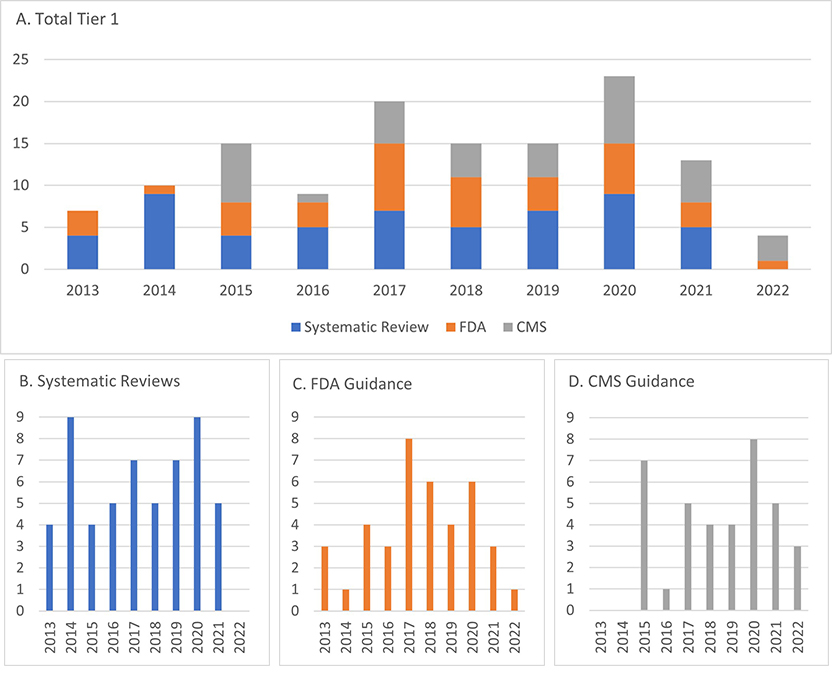
Trends over time for Tier 1 guidelines are presented in Figure 1, which displays the number of guideline documents by year.
Figure 1. Trend by year (2013- July 2022) of A) Tier 1 overall guidance documents and based on systematic review, B) FDA companion diagnostic devices or genetic testing requirements based on drug labeling, and C) CMS coverage determinations.
Systematic Review Guideline (SR) Documents
Reviewing guideline documents Overall, cancer was the focus of the majority of Tier 1 SR documents (n=40, 71%). Hereditary breast and ovarian cancer were most common (n=17), followed by colorectal cancer and Lynch syndrome guidelines (n=8) and lung cancer (n=6).
Within SR documents addressing germline testing, predictive risk testing accounted for the majority (n=12), followed by genetic counseling (n=11) and medical management (n=11).
For SR documents addressing somatic testing, purpose was mostly pharmaceutical management (n=19).
FDA companion diagnostic device or label requiring use of test to inform choice or dose of drug
FDA guidance documents heavily featured somatic testing (n=24). All Tier 1 FDA documents were related to cancer (n=36), except for a few related to abnormal blood conditions (n=2), and one related to obesity. Pharmaceutical management was the genomic application focus in all FDA guideline documents.
CMS coverage for diagnosis or treatment of reasonable and necessary items/services
The majority of CMS coverage determinations also addressed somatic testing (n=25). Germline testing was addressed in nine determinations, and both somatic and germline was addressed in two determinations. In addition to the somatic and germline testing, there was one related to karyotyping.
Diagnostic strategies were found to be the most common genomic purpose within the CMS determinations (n=19), followed by pharmaceutical management (n=17) and prognosis (n=15). Surgical management (n=5), risk management (n=4), predictive testing (n=3) and medical management (n=2) rounded out this group.
Why is it important to track Tier 1 genomic applications for health care and population health?
We encourage our readers to peruse in the database for more findings of interest. Overall, assigning tier levels to the guideline documents in the CDC Tier-Classified Guidelines Database can quickly inform interested stakeholders such as providers, policy makers and the public of the existence of guidelines in genomic medicine. If implemented widely at the population level, these guidelines can actually save lives and prevent various diseases.
Our recent paper highlighted the inequities around implementation of Tier 1 genomic applications and provided detail for three specific conditions including familial hypercholesterolemia, Lynch syndrome, and hereditary breast and ovarian cancer. Examples of inequities included lack of insurance coverage, health care providers failing to discuss relevant genetic testing with their patients, lack of alignment between clinical guidelines and uptake of risk reducing surgery. Highlighting Tier 1 guideline documents as they become available is integral in improving health outcomes in all segments of the population.
As more genetic discoveries lead to additional Tier 1 applications, we will continue to update the database. We plan to standardize processes for systematically evaluating evidence on the implementation of Tier 1 genomic applications in different communities. Considerations for implementation specific to communities and populations negatively affected by social determinants of health could help multilevel implementation research with an important goal to reduce health disparities in the implementation of genomic medicine.
Posted on by