Precision Medicine in Action: How well does cascade screening for hereditary conditions work in the real world?
Posted on by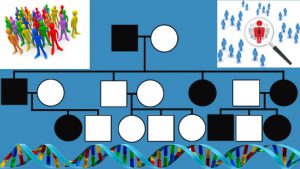 An important component of precision medicine is the identification, through genetic testing, of people who are at elevated risk of disease because of pathogenic germline mutations. Cascade screening involves contacting relatives of patients with certain hereditary conditions to help inform, manage, and identify those who may be at increased risk. A systematic scoping review on cascade screening, authored by members of the Genomics and Population Health Action Collaborative (GPHAC), is featured in the May 2018 issue of Health Affairs focused on precision medicine. Scoping reviews are intended to provide a high-level overview of a topic, using qualitative and descriptive means rather than quantitative synthesis of outcomes. The GPHAC scoping review presents a broad overview of cascade screening interventions, facilitators, barriers, relevant policy considerations, and future research needs. Relevant peer-reviewed literature from the period 1990-2017 was searched, and 122 articles identified for inclusion in the review. Included studies represented 25 different disorders, the most common of which were familial hypercholesterolemia (35 studies), hereditary breast and ovarian cancer (16 studies), and Lynch syndrome (14 studies), 3 CDC tier 1 genomic applications. Forty-five statutes and regulations related to the use and release of genetic information across the 50 states were also described in the review. The authors sought, but did not identify, standardized best practices to optimize cascade screening across geography and policy contexts. Studies that involved trained providers contacting relatives directly, rather than contacting them through the probands (or index patients), demonstrated improved cascade screening uptake; however, policies in some states might limit this approach. The literature suggests that cascade screening might be a cost-effective way to find people with particular hereditary disorders in some health care settings, but many existing programs have suboptimal uptake among relatives, thus reducing effectiveness. Substantial barriers to delivery of cascade screening included less than optimal communication with the proband and family, and geographic barriers to accessing genetic services. Not many US-based studies assessed interventions for cascade screening or used rigorous study designs, such as randomized controlled trials. In sum, this scoping review did not find standardized processed to optimize cascade screening across geographic regions and policy context. There is an urgent need for rigorous implementation studies on cascade screening in diverse populations, that also account for state policy considerations. Future studies that more rigorously assess the implementation of cascade screening programs may be helpful in finding the most effective delivery strategies. This work will be important in moving the field of precision medicine forward by identifying high-risk people and connecting them to health services that can save lives and prevent disease.
An important component of precision medicine is the identification, through genetic testing, of people who are at elevated risk of disease because of pathogenic germline mutations. Cascade screening involves contacting relatives of patients with certain hereditary conditions to help inform, manage, and identify those who may be at increased risk. A systematic scoping review on cascade screening, authored by members of the Genomics and Population Health Action Collaborative (GPHAC), is featured in the May 2018 issue of Health Affairs focused on precision medicine. Scoping reviews are intended to provide a high-level overview of a topic, using qualitative and descriptive means rather than quantitative synthesis of outcomes. The GPHAC scoping review presents a broad overview of cascade screening interventions, facilitators, barriers, relevant policy considerations, and future research needs. Relevant peer-reviewed literature from the period 1990-2017 was searched, and 122 articles identified for inclusion in the review. Included studies represented 25 different disorders, the most common of which were familial hypercholesterolemia (35 studies), hereditary breast and ovarian cancer (16 studies), and Lynch syndrome (14 studies), 3 CDC tier 1 genomic applications. Forty-five statutes and regulations related to the use and release of genetic information across the 50 states were also described in the review. The authors sought, but did not identify, standardized best practices to optimize cascade screening across geography and policy contexts. Studies that involved trained providers contacting relatives directly, rather than contacting them through the probands (or index patients), demonstrated improved cascade screening uptake; however, policies in some states might limit this approach. The literature suggests that cascade screening might be a cost-effective way to find people with particular hereditary disorders in some health care settings, but many existing programs have suboptimal uptake among relatives, thus reducing effectiveness. Substantial barriers to delivery of cascade screening included less than optimal communication with the proband and family, and geographic barriers to accessing genetic services. Not many US-based studies assessed interventions for cascade screening or used rigorous study designs, such as randomized controlled trials. In sum, this scoping review did not find standardized processed to optimize cascade screening across geographic regions and policy context. There is an urgent need for rigorous implementation studies on cascade screening in diverse populations, that also account for state policy considerations. Future studies that more rigorously assess the implementation of cascade screening programs may be helpful in finding the most effective delivery strategies. This work will be important in moving the field of precision medicine forward by identifying high-risk people and connecting them to health services that can save lives and prevent disease.
Source: Megan C. Roberts, W. David Dotson, Christopher S. DeVore, Erica M. Bednar, Deborah J. Bowen, Theodore G. Ganiats, Ridgely Fisk Green, Georgia M. Hurst, Alisdair R. Philp, Charité N. Ricker, Amy C. Sturm, Angela M. Trepanier, Janet L. Williams, Heather A. Zierhut, Katherine A. Wilemon, and Heather Hampel. Delivery Of Cascade Screening For Hereditary Conditions: A Scoping Review Of The Literature. Health Affairs. May 8, 2018.
Posted on by


