Public Health Impact of Digital Health: Reinventing the Wheel
Posted on by “Digital health has potential to improve health management, but the current state of technology development and deployment requires a “buyer beware” cautionary note.” (Perakslis and Ginsburg, JAMA, 2020)
“Digital health has potential to improve health management, but the current state of technology development and deployment requires a “buyer beware” cautionary note.” (Perakslis and Ginsburg, JAMA, 2020)
In a recent JAMA viewpoint, Perakslis and Ginsburg summarize the current state of digital health and discuss approaches in evaluating benefits, risks and value of these technologies.
Digital health includes numerous measurement technologies such as personal wearable devices, internal devices, and sensors that could be used to identify health status and help with disease diagnosis and management. Perhaps most importantly, digital health technologies provide new approaches to capture continuous data on individuals and populations that enhance our current irregular and episodic health care approaches (pulse, blood pressure, etc.).
At the outset, the authors admit that the evidence base in support of digital health is still in its infancy. There are no clinical guidelines that currently incorporate digital health tools. Few digital health technologies have been shown to be equivalent to accepted measurement tools. Only a few clinical trials are ongoing to assess the clinical utility of digital health.
Evaluating Digital Health
From our perspective, the evaluation of digital health technologies is not that different than the evaluation of new genomic tests. To the extent a digital technology is measuring a disease or health state in a person or a community, it can be viewed as a “test’ and thus subject to evaluation of its analytic and clinical validity and clinical utility with observational studies and randomized trials. Obviously, digital health technologies involve more than results of a test but do involve other aspects such as the movement of existing health-related data across health systems, patients and populations. Those attributes are not discussed here.
Just like genomic tests, electronic medical records, mobile health apps, and wearable devices and sensors contribute to new health related data. These data require standardization, evaluation for validity and health outcomes, and integration across health information technology systems. Just like genomic tests, digital health tools also come with ethical challenges. An important outcome of the massive amount of information that can be gleaned are the competing needs for security and privacy, especially in the contexts of clinical monitoring and public health surveillance. Also, new digital health technologies powered by machine learning/artificial intelligence can exacerbate existing racial and ethnic disparities due to measurement biases, as well as differential access to the technologies.
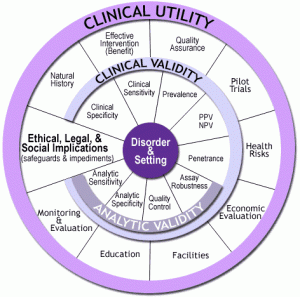 For more than a decade, our office sponsored the EGAPP initiative (Evaluation of Genomic Applications in Practice and Prevention), an evidence-based approach to assess the benefits and harms of new genetic tests in treatment and prevention. EGAPP’s methodology was founded on the ACCE model that we have used to evaluate the analytic validity, clinical validity, clinical utility and ethical, legal and social implications of genomic tests for each intended use. The ACCE “wheel” (see figure) consists of a series of 44 questions that were developed to address the rapid pace of evidence generation in genomic medicine. The ACCE wheel (with some modification of its 44 proposed questions) may provide as an appropriate template for evaluation. The attached table provides a first attempt to modify the list of questions that were devised for genomic tests. Additional work will be needed to refine the evaluation approach to digital health data.
For more than a decade, our office sponsored the EGAPP initiative (Evaluation of Genomic Applications in Practice and Prevention), an evidence-based approach to assess the benefits and harms of new genetic tests in treatment and prevention. EGAPP’s methodology was founded on the ACCE model that we have used to evaluate the analytic validity, clinical validity, clinical utility and ethical, legal and social implications of genomic tests for each intended use. The ACCE “wheel” (see figure) consists of a series of 44 questions that were developed to address the rapid pace of evidence generation in genomic medicine. The ACCE wheel (with some modification of its 44 proposed questions) may provide as an appropriate template for evaluation. The attached table provides a first attempt to modify the list of questions that were devised for genomic tests. Additional work will be needed to refine the evaluation approach to digital health data.
Understanding the full public health impact and value of digital health will take time and rigorous studies. As digital health technologies become standardized and interoperable, and as evidence of utility develops, health care and public health will become more efficient and effective, ushering the new era of precision medicine. At the same time, potential risks and harms must be addressed. Until then, digital health adoption will be a challenge to its widespread use. Just like genomic tests, there are no shortcuts on the long road to evidence-based digital health.
Posted on by


