Lab Quality Program Important to Newborn Screening
Posted on by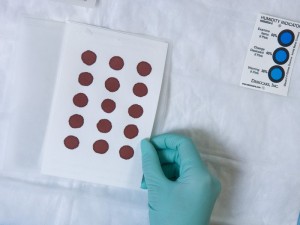 Learn about one of the nation’s most successful public health programs for newborn screening – CDC’s National Center for Environmental Health Lab Quality Program.
Learn about one of the nation’s most successful public health programs for newborn screening – CDC’s National Center for Environmental Health Lab Quality Program.
CDC’s Division of Laboratory Sciences in the National Center for Environmental Health plays an important role in newborn screening by offering the Newborn Screening Quality Assurance Program (NSQAP) to local, state, and international laboratories, and assuring newborn screening test results are as accurate as possible.
Shortly after a baby is born, a health professional takes a few drops of blood from the baby’s heel. The blood sample is sent to a state laboratory to be analyzed for several severe disorders. This process, known as newborn screening, is one of the nation’s most successful public health programs because the early identification of severe disorders has led to timely intervention and life-saving treatments for newborn children. A big part of the success of newborn screening in the laboratory is CDC’s NSQAP. This voluntary, non-regulatory program helps state health departments and their laboratories maintain and enhance the quality of test results.

Joanne Mei leads NSQAP in the Newborn Screening and Molecular Biology Branch in CDC’s Division of Laboratory Sciences, National Center for Environmental Health. The program’s role, she says, is simple: “We exist to help newborn screening labs minimize the risk of making errors.”
“We make about a million dried blood spots every year,” says Mei. “This work starts in the early morning, with people preparing the filter paper cards. We lay the cards out on a rack, and a robot spots the blood onto them. We add different biochemical markers depending on what newborn screening disorder we’re trying to mimic.”
More than 650 newborn screening laboratories across the U.S. and the world depend on these specially prepared cards, known as dried blood spot quality assurance samples, to provide an “external check” on their work.
The quality assurance samples are sent to participating laboratories to see if they arrive at the expected answer, or identify the biochemical marker for a newborn screening disorder. If a participating laboratory does not get the correct answer, NSQAP immediately works with the laboratory to identify and solve the problem.
“The labs use our materials on a routine basis,” says Mei. “For example, we send out blinded samples every few months, and the labs test those samples and send us back the results. In newborn screening, the labs are trying to capture every baby who could have a particular disorder.”
When a new disorder is recommended for newborn screening, developing appropriate dried blood spot quality assurance samples can take years. “We work with the states [that want to screen for the new disorder] and with manufacturers who are developing new screening tests,” says Mei. “We have to make sure the biochemical marker for that disease is going to be detected on the filter paper and is going to be stable on the filter paper over time.” The critical goal is “to manufacture blood spots with levels of biomarkers that look like a baby with disease.”
“If it’s a recommended newborn screening blood spot test,” says Mei, “we have materials for it.” No other entity in the world can match the level of support the CDC team provide.
A Snapshot of Newborn Screening
Shortly after a baby is born, a health professional takes a few drops of blood from the baby’s heel. The blood sample is sent to a state laboratory to be analyzed for several severe disorders. This process, known as newborn screening, is one of the nation’s most successful public health programs because the early identification of severe disorders has led to timely intervention and life-saving treatments for newborn children.
More Information
Newborn Screening Quality Assurance Program
Newborn Screening and Molecular Biology Branch
Tweet this: “One of the nation’s premier public health programs for newborn screening. http://bit.ly/2cpTDlK #CDCEHblog via @CDCEnvironment”


Post a Comment