Antigen Testing for SARS-CoV-2 in Non-healthcare Workplaces
Posted on by
The health of workers and businesses’ success during the COVID-19 pandemic rely on effective workplace prevention and control measures. In a recent commentary in the Journal of Occupational and Environmental Medicine, researchers from the National Institute for Occupational Safety and Health discussed the use of antigen testing in the workplace. Antigen testing (as well as all viral tests) can identify people with COVID-19 who are not showing signs or symptoms and who have no known contact with people who have infection with SARS-CoV-2, the virus that causes COVID-19. These antigen tests can be an effective tool to help employers prevent the spread of SARS-CoV-2, the virus that causes COVID-19, in their workplace. Antigen tests are relatively inexpensive, can be easily administered in the workplace or at home by a healthcare provider or the worker, and provide results within minutes. NIOSH researchers also provided a framework (see Figure 2) for using antigen tests to identify SARS-CoV-2 infections in the workforce.
Vaccination remains the leading public health prevention strategy to end the COVID-19 pandemic by reducing transmission, and significantly reducing the risk of severe disease, hospitalization and death. In addition to vaccination, workplaces can follow the hierarchy of controls to protect against COVID-19 in the workplace. This involves using engineering controls, such as partitions or ventilation, combined with administrative controls, such as physical distancing, hand washing, and in some situations, personal protective equipment and masks [1]. Screening for the virus with either nucleic acid amplification tests (NAATs) or antigen tests is an effective administrative control for early detection of individuals with COVID-19 and allows employers to take steps to prevent workplace spread.
While viral testing using the more sensitive NAATs usually requires laboratory testing, rapid antigen tests that require fewer resources can play an important role in efforts to stop the spread of SARS-CoV-2 in the workplace.
These rapid antigen tests are being used widely in schools, at various mass gatherings, and by sports organizations [2-5]. Worldwide, nearly 100 companies are developing or manufacturing tests for antigen detection [6-7]. Antigen tests with emergency use authorization from the U.S. Food and Drug Administration are listed here.
CDC guidance recommends that serial (or regular) antigen testing for screening unvaccinated workers is an effective way to prevent the spread of SARS-CoV-2 in the workplace. Low test positivity may indicate that prevention strategies in place are effective and may factor into decisions for a safe return to in-person learning and work. Rising case rates can serve as an early warning sign that prevention strategies should be strengthened or added in the business and larger community. In addition, screening testing can help stop spread by quickly identifying cases that show no symptoms, which are estimated to be the source for at least one half of the spread of infections [8-9]. See Figure 1.
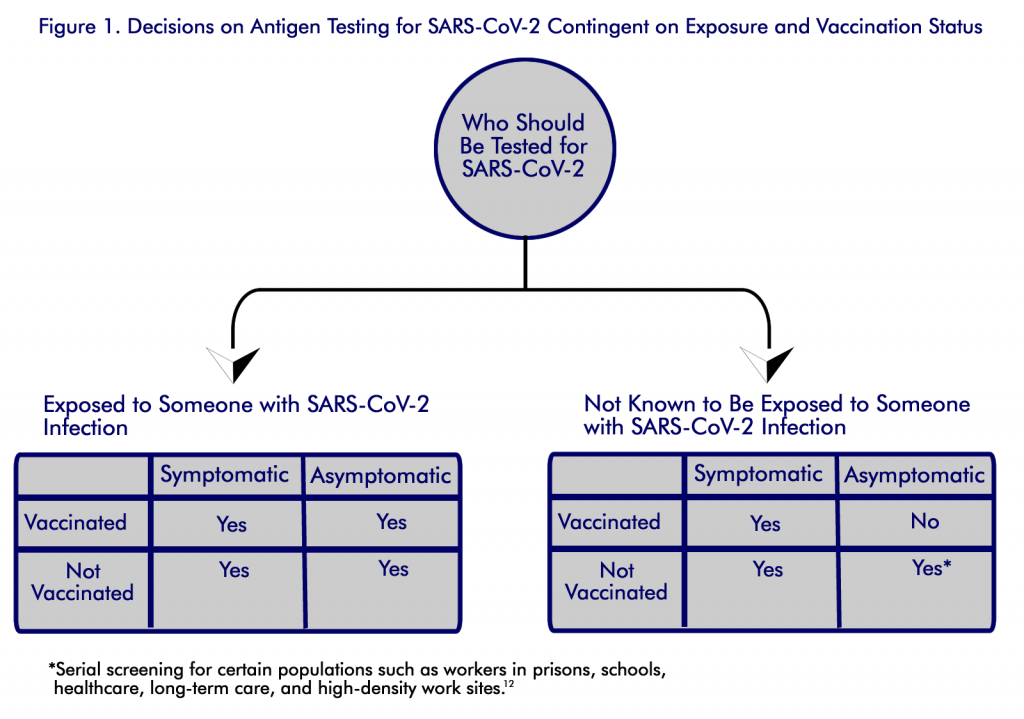
With rapid detection, persons with infection can be isolated, and contact tracing and quarantine can be used to control further spread [10]. The CDC recommends that unvaccinated persons without symptoms or exposure in certain groups should receive regular screening. The guidance recommends screening the following groups as especially useful due to their high risk of exposure or severe illness: students and staff members of kindergarten–grade 12 schools and institutions of higher education, healthcare personnel, residents and staff members of long-term care facilities, persons in prison, persons experiencing homelessness, and workers in high-density worksites [10] .
A Proposed Framework for Antigen Testing
Programs that use antigen testing for screening workers, along with other preventive measures, can help support a healthy workplace [10]. Identifying active cases of COVID-19 among workers can help stop transmission and contribute to ending the pandemic [11].
The framework proposed by NIOSH researchers and based on scientific literature recommends that employers consider using an antigen test screening program. For specific steps, see the commentary for detailed information. Employers may choose to use antigen testing with regular frequency, before allowing workers to enter the workplace, while at work (including travel for work), or when returning to work after exposure or self-initiated isolation.
An important trigger for initiating testing is a substantial or high burden of COVID-19 in the community. Other triggers could include having an outbreak in the workforce, having workers who are at high risk of severe COVID-19 illness, or wanting to ensure public confidence in the safety of the workplace as a critical component of business operations.
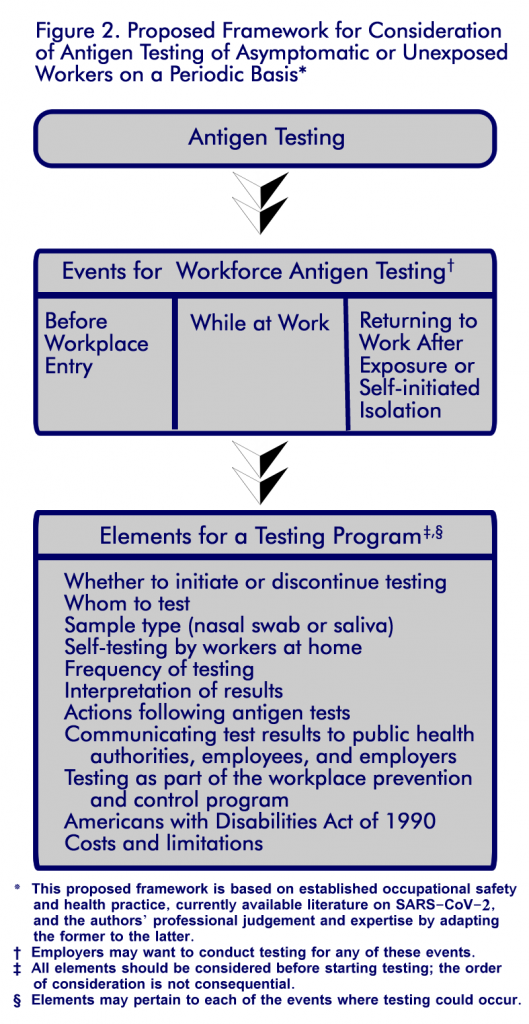 The elements of the framework are shown in Figure 2.
The elements of the framework are shown in Figure 2.
A viral testing program is most useful as part of a comprehensive plan that includes strategies for communicating with workers and plans for changing operations on the basis of test results when needed [12]. Communicate to workers that antigen tests in the workplace do not provide a clinical diagnosis but detect persons who are infected and may spread the virus to others, even if they are not experiencing symptoms or have a known or suspected exposure to COVID-19. Subsequent testing would confirm or negate the initial test.
An antigen testing program is likely to be successful if the employer has the capability and capacity to conduct the testing either directly or through contractors and consultants [12,13]. Although all workers could be the focus of testing, this framework focuses on unvaccinated workers without symptoms and workers without a known exposure who are at increased risk. For example, this includes people who work in close proximity of each other or with customers and suppliers [12,13,14].
Following the Test
Workers who show no symptoms and unexposed workers who receive a negative antigen test can be allowed to enter the workplace and work. Workers who test positive should follow CDC recommendations regarding isolation, not reporting to work, and contacting their personal healthcare provider for appropriate management [14,15]. CDC has encouraged employers to consider implementing flexible, non-punitive paid sick leave and supportive policies and practices [10,12].
CDC guidance recommends a worker who tests positive but is not showing symptoms of COVID-19 should be excluded from work and should isolate. The employer should also support contact tracing activities by public health professionals for workers who work near workers who test positive [13,14,16,17]. The employer should have means to keep workers’ test data confidential [18]. Reporting of test results to state or local public health departments is mandated by the Coronavirus Aid, Relief, and Economic Security (CARES) Act [19].
Challenges in Using This Framework
The ability to provide rapid antigen testing for workers will depend on the availability of tests. The ability to test regularly and interpret results in a timely fashion are critical to the effective use of antigen tests. Another important limitation is whether results of antigen tests that are self-administered by workers will be provided to appropriate local health authorities and counted in community statistics [20].
The costs of antigen tests are variable but are generally estimated to be $5 to $50 [14, 21]. Kits that can be used by workers at home are typically lower in cost range. Some issues regarding the use of antigen testing are not completely resolved such as who pays for the tests and related costs and when and how to administer tests. Resources such as the When to Test calculator may be helpful. **
Vaccination is the leading public health prevention strategy to end the COVID-19 pandemic. Antigen testing is a useful public health tool that employers can use to stop the spread in the workplace. Has your workplace used antigen testing? Please share what has worked well and any challenges you faced in the comment section below.
Paul A. Schulte, PhD, is Director of the NIOSH Division of Science Integration.
Marie A. de Perio, MD, is the Senior Medical Advisor in the NIOSH Office of the Director.
Sophia K. Chiu, MD, is a Medical Officer in the NIOSH Division of Field Studies and Engineering.
John D. Piacentino, MD, MPH, is the NIOSH Associate Director for Science.
David N. Weissman, MD, is Director of the NIOSH Respiratory Health Division.
Lewis J. Radonovich, MD, is a Medical Officer in the NIOSH Respiratory Health Division.
Douglas Trout, MD, MHS, is Deputy Director of the NIOSH Office of Construction Safety and Health.
Don Beezhold, PhD, is Director of the NIOSH Health Effects Laboratory Division.
Frank J. Hearl, SM, PE, is Chief of Staff in the NIOSH Office of the Director.
John Howard, MD, is Director of the National Institute for Occupational Safety and Health.
** References to products or services do not constitute an endorsement by NIOSH or the U.S. government.
References
- Centers for Disease Control and Prevention (CDC). Guidance for Business and Employers Responding to Coronavirus Disease 2019 (COVID-19). December 31, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/guidance-business-response.html. Accessed December 31, 2020.
- Association for Public Health Laboratories (APHL). Considerations of SARS-CoV-2 Rapid Antigen Testing. Version 4. September 24, 2020. Available at: https://www.aphl.org/programs/preparedness/Crisis-Management/Documents/APHL-SARSCov2-Antigen-Testing-Considerations.pdf. Accessed September 27, 2020.
- American College Health Association (ACHA). COVID-19 Weekly Updates. December 16, 2020. Available at: https://www.acha.org/ACHA/Resources/COVID-19_Novel_Coronavirus/Updates_on_COVID-19/ACHA/Resources/Topics/COVID-19_Update.aspx?hkey=3457e5ad-6492-4f6e-b07b-83226a92251. Accessed December 28, 2020.
- Wu KJ. Trump Announces Plan to Ship 150 Million Rapid Coronavirus Tests. New York Times. September 28, 2020. Available at: https://www.nytimes.com/2020/09/28/health/trump-coronavirus-testing-rapid.html?searchResultPosition=2. Accessed September 28, 2020.
- Riley C. Missouri Colleges and Universities Receive 77k+ Rapid-result COVID-19 Antigen Tests. Springfield News-Leader. Dec 26, 2020. Available at: https://www.news-leader.com/story/news/education/2020/12/27/missouri-colleges-universities-receive-77-k-covid-19-antigen-tests/4031743001/. Accessed December 28, 2020.
- World Health Organization (WHO). Antigen-detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immune Assays. Interim Guidance, September 11 2020. 2020; Geneva: World Health Organization, 1–9.
- Foundation for Innovative New Diagnostics (FIND). SARS-CoV-2 Diagnostic Pipeline. December 20, 2020. Available at: https://www.finddx.org/covid-19/pipeline/. Accessed December 22, 2020.
- Moghadas SM, Fitzpatrick MC, Sah P, et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci U S A 2020;117:17513–5. PMID:32632012
- Johansson MA, Quandelacy TM, Kada S, et al. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open 2021;4:e2035057. https://doi.org/10.1001/jamanetworkopen.2020.35057 PMID:33410879
- Christie A, Brooks JT, Hicks LA, et al. Guidance for Implementing COVID-19 Prevention Strategies in the Context of Varying Community Transmission Levels and Vaccination Coverage. MMWR Morb Mortal Wkly Rep 2021;70:1044–1047. DOI: http://dx.doi.org/10.15585/mmwr.mm7030e2
- Michaels D, Wagner GR. Halting Workplace COVID-19 Transmission: An Urgent Proposal to Protect American Workers [The Century Foundation web site]. October 15, 2020. Available at https://tcf.org/content/report/halting-workplace-covid-19-transmission-urgent-proposal-protect-american-workers/. Accessed February 16, 2021.
- Centers for Disease Control and Prevention (CDC). Interim Guidance for SARS-CoV-2 Testing in Non-healthcare Workplaces. March 17, 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/testing-non-healthcare-workplaces.html. Accessed April 12, 2021.
- Centers for Disease Control and Prevention (CDC). Overview of Testing for SARS-CoV-2 (COVID-19). March 17, 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Accessed April 12, 2021.
- Centers for Disease Control and Prevention (CDC). Interim Guidance for Antigen Testing for SARS-CoV-2. December 16, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html. Accessed December 16, 2020.
- Centers for Disease Control and Prevention (CDC). Discontinuation of Isolation for Persons with COVID-19 not in Healthcare Settings. February 18, 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patients.html. Accessed April 12, 2021.
- Centers for Disease Control and Prevention (CDC). COVID-19 Case Investigation on Contact Tracing in Non-Healthcare Workplaces. October 16, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/contact-tracing-nonhealthcare-workplaces/FS-Employers.html. Accessed October 16, 2020.
- Centers for Disease Control and Prevention (CDC). Case Investigation and Contact Tracing in Non-healthcare Workplaces: Information for Employers. October 22, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/contact-tracing-nonhealthcare-workplaces.html. Accessed October 22, 2020.
- Centers for disease Control and Prevention (CDC). Workplace SARS-CoV-2 Testing: Consent Elements and Disclosures. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/workplaces-businesses/workplace-testing-consent-elements-disclosures.html. Accessed March 1, 2021.
- Department of Health and Human Services (DHHS). COVID-19 Pandemic Response, Laboratory Data Reporting: CARES Act Section 18115. January 8, 2021. Available at: https://www.hhs.gov/sites/default/files/covid-19-laboratory-data-reporting-guidance.pdf. Accessed January 9, 2021.
- Pettengill MA, McAdam AJ. Can we test our way out of the COVID-19 pandemic? J Clin Microbiol2020; 58:e02225–e2320.
- Abbott Laboratories. Abbott’s Fast, $5, 15-minute, Easy-to-use COVID-19 Antigen Test Receives FDA Emergency Use Authorization; Mobile App Displays Test Results to Help Our Return To Daily Life; Ramping Production to 50 Million Tests a Month. August 26, 2020. Available at: https://abbott.mediaroom.com/2020-08-26-Abbotts-Fast-5-15-Minute-Easy-To-Use-COVID-19-Antigen-Test-Receives-FDA-Emergency-Use-Authorization-Mobile-App-Displays-Test-Results-to-Help-Our-Return-to-Daily-Life-Ramping-Production-to-50-Million-Tests-a-Month. Accessed August 30, 2020.
7 comments on “Antigen Testing for SARS-CoV-2 in Non-healthcare Workplaces”
Comments listed below are posted by individuals not associated with CDC, unless otherwise stated. These comments do not represent the official views of CDC, and CDC does not guarantee that any information posted by individuals on this site is correct, and disclaims any liability for any loss or damage resulting from reliance on any such information. Read more about our comment policy ».
I think there is not enough evidence for it. The best indication is to test asymptomatic workers when they are in contact with a confirmed case, even if they are vaccinated. In the company where I work, the screening of respiratory symptoms and contacts has been adequate. We only use tests on respiratory symptoms workers. Only if the traceability shows that the confirmed case did not use a mask, antigen tests are carried out on the all contacts. That was enough to control eventual outbreaks.
Thanks for reporting your experience.
At my workplace, all employees are required to perform Covid-19 test using the saliva self-test on biweekly basis. Recently we had several employees with positive test results. To confirm the results, we sent them for another round of testing using PCR tests and they turned out to be negative. This trigger a question … would the negative results using saliva tests turn positive on PCR?
Thank you for your comment. It is possible to get a positive PCR test following a negative antigen test since the RT-PCR test is more sensitive in detecting an infection.
The FDA has commented that some false positive results can occur with antigen tests: https://www.fda.gov/medical-devices/letters-health-care-providers/potential-false-positive-results-antigen-tests-rapid-detection-sars-cov-2-letter-clinical-laboratory. As noted by FDA, “Laboratories should expect some false positive results to occur even when very accurate tests are used for screening large populations with a low prevalence of infection.” FDA also notes the importance of carefully following instructions for performing antigen tests and considering confirming positive results with RT-PCR tests within 48 hours, especially in low-incidence counties.
FDA also touches on the topic of your question, whether false negative tests can occur. FDA notes that “In general, antigen tests are not as sensitive as molecular tests. Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions. Negative results from an antigen test should be considered in the context of clinical observations, patient history and epidemiological information.”
What a great article. In my company we have been taking the temperature for months and doing these tests to prevent more infections.
This is a good article. Does it inform policy put forth by other federal agencies such as OSHA? Mitigation strategies for reducing spread of SARS Co-V-2 in the work place needs to be multilayered: vaccination, masking and improved indoor air quality (through increased ventillation and/or air disinfection). No single strategy seems perfect, which is why using all effective measures can give us the best chance to move past this epidemic phase to the phase when this virus remains amongst us as an endemic infection.
Thank you for your comment and question. CDC recommends workplaces implement layered prevention strategies, which include vaccination, ventilation, testing, hand hygiene, and mask use. Throughout the pandemic, we have been working with other federal partners to share the science and guide decision making.