Nanotechnology Research at NIOSH
Posted on by
Engineered nanomaterials (ENMs) are materials that are intentionally produced to have at least one primary dimension less than 100 nanometers. These materials have new or unique properties different from those of larger forms of the same material, making them desirable for specific product applications. These properties can contribute to increased elasticity, tensile strength, electrical conduction, and reactivity. Consumer products using nanomaterials include makeup, sunscreen, food storage products, appliances, clothing, electronics, computers, sporting goods, and coatings.
The health effects associated with the wide variety of ENMs are not yet clearly understood, so it is important for producers and users of engineered nanomaterials to reduce employee exposure and manage risks appropriately. NIOSH has been at the forefront of efforts to characterize the potential hazards for those working with ENMs and ensure safe workplaces. Since 2004, NIOSH has led the federal government health and safety initiative for nanotechnology, with research and activities coordinated through the NIOSH Nanotechnology Research Center (NTRC). NIOSH has published numerous publications providing guidance on nanomaterial worker health and safety, including guidance for research laboratories and small businesses, and risk assessment documentation and recommended exposure limits for nanoscale titanium dioxide, carbon nanotubes, and carbon nanofibers. NIOSH has also created a field studies team to assess workplace processes, materials, and control technologies associated with nanotechnology and additive manufacturing processes including 3D printing. Since 2004, this team has provided over 100 cost-free, on-site exposure assessments at a variety of ENM and advanced manufacturing facilities.
Protecting Workers
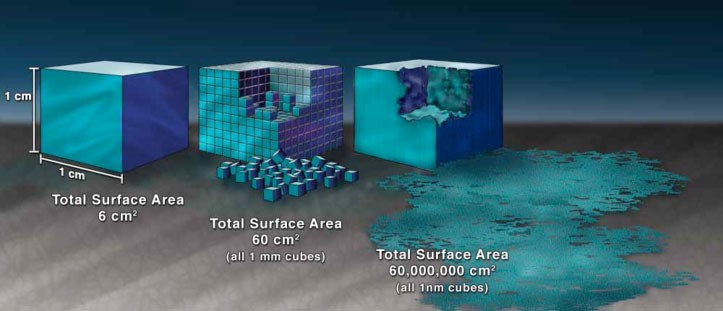
Research over the past 20 years shows that (1) small particles, on an equal mass basis, can be more hazardous than larger ones; (2) free, un-bound ENMs that become airborne can be inhaled and deposited in the smallest and deepest areas of the lungs and some can move to other organs (Elder & Oberdörster, 2006; Oberdörster et al., 2009; Yu et al., 2017); (3) ultrafine particles have been linked to respiratory (such as lung inflammation and fibrosis) and cardiovascular health effects (Farina et al. 2019; Frampton 2001; Zhao et al., 2019); and (3) certain high volume ENMs, such as ultrafine titanium dioxide and carbon black are respiratory hazards (IARC 2010).
It is recommended that employers inform their workers about the dangers of nanomaterial hazards in their work-places and train their employees on proper safeguards. Many hazard controls are available for engineered nanomaterials. When addressing workplace hazards, it is recommended to follow the hierarchy of controls. Elimination and substitution may be accomplished by changing a nanomaterial’s size, shape, functionalization, surface charge, solubility, agglomeration, or aggregation state to improve its toxicological properties while maintaining the desired properties and functionality. As one of the primary routes for exposure is via inhalation, another example is substituting a nanomaterial slurry instead of using a 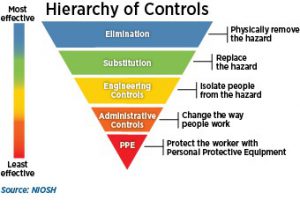 dry powder to reduce the potential for aerosolization. Examples of engineering controls, which isolate or contain the hazard, include fume hoods and glove boxes exhausted through a HEPA filter, continuous-feed bag liners, and walk-off sticky mats. Administrative controls include good housekeeping practices such as hand-washing, wet-wiping cleanup, and use of HEPA-filtered vacuums; training employees; limiting the time the workers handle the material; and implementing proper labeling and storage of materials. Personal protective equipment, although not recommended as primary worker protection, can include long pants without cuffs and a long-sleeved shirt; non-cotton laboratory coats or coveralls; nitrile or other chemically impervious gloves; closed-toe shoes made of a low-permeability material such as leather, or disposable over-the-shoe booties; safety glasses, safety goggles and/or face shields; and fit-tested respirators.
dry powder to reduce the potential for aerosolization. Examples of engineering controls, which isolate or contain the hazard, include fume hoods and glove boxes exhausted through a HEPA filter, continuous-feed bag liners, and walk-off sticky mats. Administrative controls include good housekeeping practices such as hand-washing, wet-wiping cleanup, and use of HEPA-filtered vacuums; training employees; limiting the time the workers handle the material; and implementing proper labeling and storage of materials. Personal protective equipment, although not recommended as primary worker protection, can include long pants without cuffs and a long-sleeved shirt; non-cotton laboratory coats or coveralls; nitrile or other chemically impervious gloves; closed-toe shoes made of a low-permeability material such as leather, or disposable over-the-shoe booties; safety glasses, safety goggles and/or face shields; and fit-tested respirators.
Exposure assessment and control verification can be performed using the Nanomaterial Exposure Assessment Method (NEAT 2.0) which includes performing a complete exposure assessment using an array of industrial hygiene methods, such as particle counters to indicate the real-time quantity of particles present and filter-based samples to identify the presence of the nanomaterial (Brenner et. al. 2016; Eastlake et. al. 2016).
Current and Future Research Activities
The NTRC identifies ENMs in commerce through market forecasting and research, technology surveillance, and connections with both domestic and international partners and stakeholders. Using this information, we can prioritize ENMs for research and focus on those materials that have the greatest occupational risk potential. Laboratory research is performed to expand our knowledge of the biological mechanisms and health effects of ENMs. This research builds the foundation for both field investigations and epidemiological research that provide a real-world understanding of the potential risk for ENM workers. Field investigations allow for face-to-face task-based feedback directly to companies that are uncertain about the potential adverse effects of ENMs. This can include guidance on engineering controls and personal protective equipment to mitigate exposure potential. Guidance provided by our combined field and laboratory research can provide ENM businesses with guidance they can use to keep workers safe, develop public trust, and accelerate their commercialization. NTRC research provides input into both national and international strategies to address health and safety of ENM workers. Although we have focused primarily on nanomaterials since 2004, within the past few years we have broadened our research and field interests and started applying our existing knowledge to advanced materials and processes, such as additive manufacturing and 3-dimensional printing.
Assessing Our Impact
The number of industries and occupational settings that use nanotechnology and ENMs continues to expand and diversify and, with that, the potential for workers to be exposed to these materials in the workplace is increasing. With this increase in exposure potential, the NTRC needs to build on our existing strategic plan and prioritize research in order to provide the most current guidance and recommendations to protect nanomaterial workers. As part of this research, RTI International is releasing a survey on behalf of NIOSH to gain information about companies’ safety and health practices surrounding the use of ENMs and how NIOSH has and could better contribute to the process.
Adrienne Eastlake, MS, RS/REHS, MT (ASCP), is an industrial hygienist in the Nanotechnology Research Center within the NIOSH Division of Science Integration Emerging Technologies Branch.
John P. Sadowski, Ph.D., is a technical analyst (contractor) in the NIOSH Office of the Director.
References
Brenner, S. A., Neu-Baker, N. M., Eastlake, A. C., Beaucham, C. C., & Geraci, C. L. (2016). NIOSH field studies team assessment: Worker exposure to aerosolized metal oxide nanoparticles in a semiconductor fabrication facility. Journal of Occupational and Environmental Hygiene, 13(11), 871-880.
Eastlake, A. C., Beaucham, C., Martinez, K. F., Dahm, M. M., Sparks, C., Hodson, L. L., & Geraci, C. L. (2016). Refinement of the nanoparticle emission assessment technique into the nanomaterial exposure assessment technique (NEAT 2.0). Journal of occupational and environmental hygiene, 13(9), 708-717.
Elder, A., & Oberdörster, G. (2006). Translocation and effects of ultrafine particles outside of the lung. Clinics in occupational and environmental medicine, 5(4), 785-796.
Farina, F.; Lonati, E.; Milani, C.; Massimino, L.; Ballarini, E.; Donzelli, E.; Crippa, L.; Marmiroli, P.; Botto, L.; Corsetto, P.A.; Sancini, G.; Bulbarelli, A.; Palestini, P. (2019). In Vivo Comparative Study on Acute and Sub-acute Biological Effects Induced by Ultrafine Particles of Different Anthropogenic Sources in BALB/c Mice. Int. J. Mol. Sci. 20, 2805.
Frampton MW. (2001) Systemic and cardiovascular effects of airway injury and inflammation: Ultrafine particle exposure in humans. Environ Health Perspect, Aug;109 Suppl 4:529-32
IARC. (2010). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 93, Carbon Black, Titanium Dioxide, and Talc. IARC, Lyon 2010; 93:1-413.
Oberdörster, G., Elder, A., & Rinderknecht, A. (2009). Nanoparticles and the brain: cause for concern?. Journal of nanoscience and nanotechnology, 9(8), 4996-5007.
Yu Y, Duan J, Li Y, Li Y, Jing L, Yang M, Wang J, Sun Z. (2017) Silica Nanoparticles induce liver fibrosis via TGF-β1/Smad3 pathway in ICR mice. Int J Nanomedicine. 12:6045-6057.
Zhao L, Zhu Y, Chen Z, Xu H, Zhou J, Tang S, Xu Z, Kong F, Li X, Zhang Y, Li X, Zhang J & Jia G (2018) Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles, Nanotoxicology, 12:2, 169-184.
2 comments on “Nanotechnology Research at NIOSH”
Comments listed below are posted by individuals not associated with CDC, unless otherwise stated. These comments do not represent the official views of CDC, and CDC does not guarantee that any information posted by individuals on this site is correct, and disclaims any liability for any loss or damage resulting from reliance on any such information. Read more about our comment policy ».
extraordinary information about nanotechnology.
I want a to be the first person with a nano suit